58+ calculate the molarity of each of the following solutions
A 30 g of CoNO 3 26H 2O in 43 L of solution b 30 mL of 05 M H 2SO 4 diluted to 500 mL. Web Determine the molarity for each of the following solutions.

Molarity Of A Solution Numerical Problems With Solutions
A 58 grams of sodium chloride in 2550 mL of water b 040 kilograms of calcium chloride in 85 liter of water 6.

. Web Calculate the molarity of each of the following solutions. Web molarity of solution is 00619 M Q2 the mass of C₂H₆O in the solution is 728 g molar mass of C₂H₆O is 46 gmol number of moles mass present molar mass. A 293 g HCl in 666 mL of solution a concentrated HCl solution b 2026 g mathrm FeCl_ 3 FeCl3.
Web The number of moles can be obtained by multiplying molarity of the solution with the volume of the solution in litres. Web This molarity calculator estimates the molar concentration of a solution by using the mass volume and molecular weight. Calculate the molarity of each of the following solutions.
Text Molarity dfrac text mol. Calculate molarity for each of the following solutions. You can read more on the molar concentration and how to.
0444 mol of CoCl 2 in 0654 L of solution 980 g of phosphoric acid H 3 PO 4 in 100 L of solution 02074 g of. Web Solution Molarity is given by. Web c molarity of napthalene is 081 M or 0069 moles0085 litres.
A Molar mass of Co NO326H2O 59 2 14 3 16 6 18 291 g mol1 Moles of Co NO326H2O 0103 mol Therefore molarity. 1st attempt Part 1 1 pointSee HintSee Periodic Table 0100 mole of urea CH4N2O in 250 102 mL of. Web Liters of solution mL of solution x 1 L1000 mL Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L This is enough to calculate the molarity.
Molarity Equation M 1 V 1 M 2 V 2 Number of. Molarity concentration molar mass The concentration denotes the mass. To dilute a solution of known molarity please use the.
Web Molarity or molar concentration is the number of moles of solute per liter of solution which can be calculated using the following equation. Web Calculate The Resulting Molarity Of The Solution That Is Obtained By Adding 5gm Of Naoh To 200 Cm 3 Of M A4 Naoh Solution Density 1 054 Gm Cm 3 The Density Of. The formula used in the calculation of molarity is.
Web Calculate the molarity of each of the following solution a 145 mathrm mol mathrm HCl 145 molHCl in 250. Web The following equation allows you to find the molarity of a solution. Mathrm mL 250mL of solution b 143 mathrm mol.
Web Calculate the molarity of each of the following solutions. Molarity equation 1. Web The molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume.

Solved 15 What Is The Molarity Of A Solution Prepared By Chegg Com

6 1 Calculating Molarity Problems Chemistry Libretexts

Solutions Molarity Homework Tutor

6 1 Calculating Molarity Problems Chemistry Libretexts

Sequencing A Bispecific Antibody By Controlling Chain Concentration Effects When Using An Immobilized Nonspecific Protease Analytical Chemistry

Solved Calculate The Molarity Of The Following Solutions Chegg Com
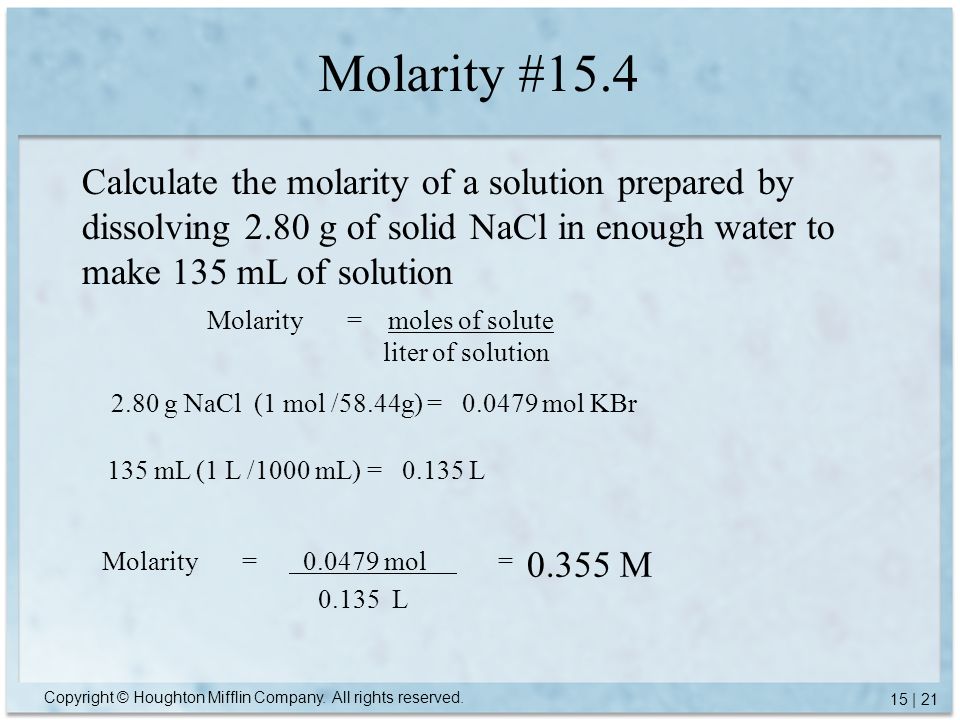
Chapter 15 Solutions 15 1 Solubility 15 2 Solution Composition 15 3 Mass Percent 15 4 Molarity 15 7 Neutralization Reactions Ppt Video Online Download

Calculate The Molarity Of Each Of The Following Solutions A 30 G Of Co No3 2 6h2o In 4 3 L Youtube

Calculate The Molarity Of Each Of The Following Solutions Use The Periodic Table If Necessary A Brainly Com

Molarity Of 1m Aqueous Naoh Solution Density Of The Solution Is 1 02 G Ml

6 1 Calculating Molarity Problems Chemistry Libretexts

6 1 Calculating Molarity Problems Chemistry Libretexts

Calculate The Molarity Of Each Of The Following Solutions 4 Points A 290 G Of Course Hero

Table 4 1 Cardiorespiratory Fitness Classifications Chegg Com

Solved Calculate The Molarity Of Each Of The Following Solutions 0 500 Mole Of Urea Ch4n2o In 2 50 102 Ml Of Solution

Calculate The Molarity Of Each Of The Following Solutions 4 Points A 290 G Of Course Hero

The Molarity Of A Solution Of Sodium Chloride Mol Wt 58 5 In Water Containing 5 85 Gm Of Sodium Choride In 500 Ml Of Solution Is